News
Lilly's GLP-1 drug Tirzepatide has been approved in china
Tirzepatide has been approved in china
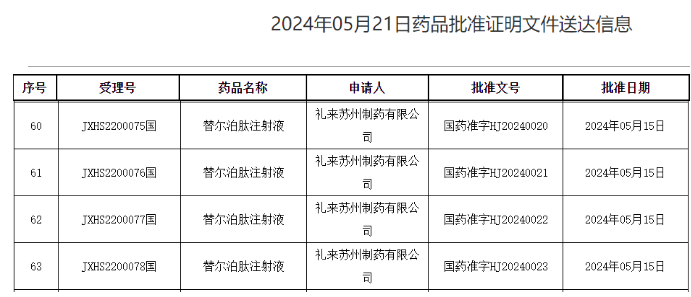
On May 21st, the official website of the National Medical Products Administration (NMPA) in China showed that Eli Lilly and Company's application for the listing of Tirzepatide injection has been approved. Tirzepatide, a GIP/GLP-1 receptor dual agonist, was approved to treat adult type 2 diabetes patients for blood glucose control.
Mufengda (Tirzepatide injection) can selectively bind and activate two natural intestinal trypsin receptors, GIP receptor and GLP-1 receptor, reducing fasting and postprandial blood glucose levels in T2DM patients. There are currently four specifications: 2.5 mg: 0.5ml, 5 mg: 0.5ml, 7.5 mg: 0.5ml, and 10 mg: 0.5ml.
This approval is mainly based on the global key phase III registration trial SURPASS 1-5 conducted in T2DM patients and the Asia Pacific key phase III registration trial SURPASS-AP Combo (83.4% of the participants are Chinese patients).
The mean baseline course of T2DM patients included in SURPASS-213 was 8.6 years, the mean HbA1c was 8.28%, the mean body weight was 93.7kg, and the mean body mass index (BMI) was 34.2kg/m2. Among them, Mufengda ® At week 40, the average decrease in HbA1c compared to baseline was 2.09% and 2.37% for subjects in the 5 mg and 10 mg treatment groups, while the average decrease in HbA1c in the 1 mg group of sitagliptin was 1.86%.
In addition, the average change in weight from baseline is one of the key secondary endpoints, Mufengda (Tirzepatide injection). The average weight loss of subjects with 5mg and 10mg was 7.8 kg and 10.3 kg, while the average weight loss of the 1mg group with semaglutide was 6.2 kg.
The average baseline course of T2DM patients included in SURPASS-AP-Combo12 was 7.7 years, the average HbA1c was 8.71%, the average weight was 76.6 kg, and the average BMI was 27.9 kg/m2. 47.5% of the subjects used sulfonylureas at baseline. Among them, Mufengda ® At week 40, the average decrease in HbA1c compared to baseline was 2.24% and 2.44% in the 5mg and 10mg treatment groups, while the average decrease in HbA1c in the glargine insulin group was 0.95%.
In addition, the average change in weight from baseline is one of the key secondary endpoints, Mufengda ® The average weight loss of the 5mg and 10mg subjects was 5.0 kg and 7.0 kg, while the average weight increase of the insulin glargine group was 1.5 kg.
The overall safety of T2DM patients in the Asia Pacific region, mainly in the Chinese population, is consistent with that of the global population, and no new safety signals have been found. Gastrointestinal reactions are the most common adverse reactions, usually occurring during the dose escalation phase and decreasing over time, with severity often ranging from mild to moderate.
CATEGORIES
News
- Retatrutide dosage chart2024-04-09
- Retatrutide's Side Effects2024-03-05
- What is retatrutide?2024-01-27
- Can retatrutide be taken orally?2024-05-07
- A competition among the top 2 GLP-1 wei2025-05-23
CONTACT US
Contact: Retatrutide online
Phone: +86 18073326374
E-mail: rose@goodpeptides.com
Add: Science and Technology Industrial Park, Yuelu District, Changsha City, Hunan Province
